|
#1
| |||
| |||
|
Can you please give me the previous year question papers of West Bengal Joint Entrance Examinations Board WBJEE? As you want to get the previous year question papers of West Bengal Joint Entrance Examinations Board WBJEE so here is the information of the same for you: Previous year question papers of West Bengal Joint Entrance Examinations Board WBJEE  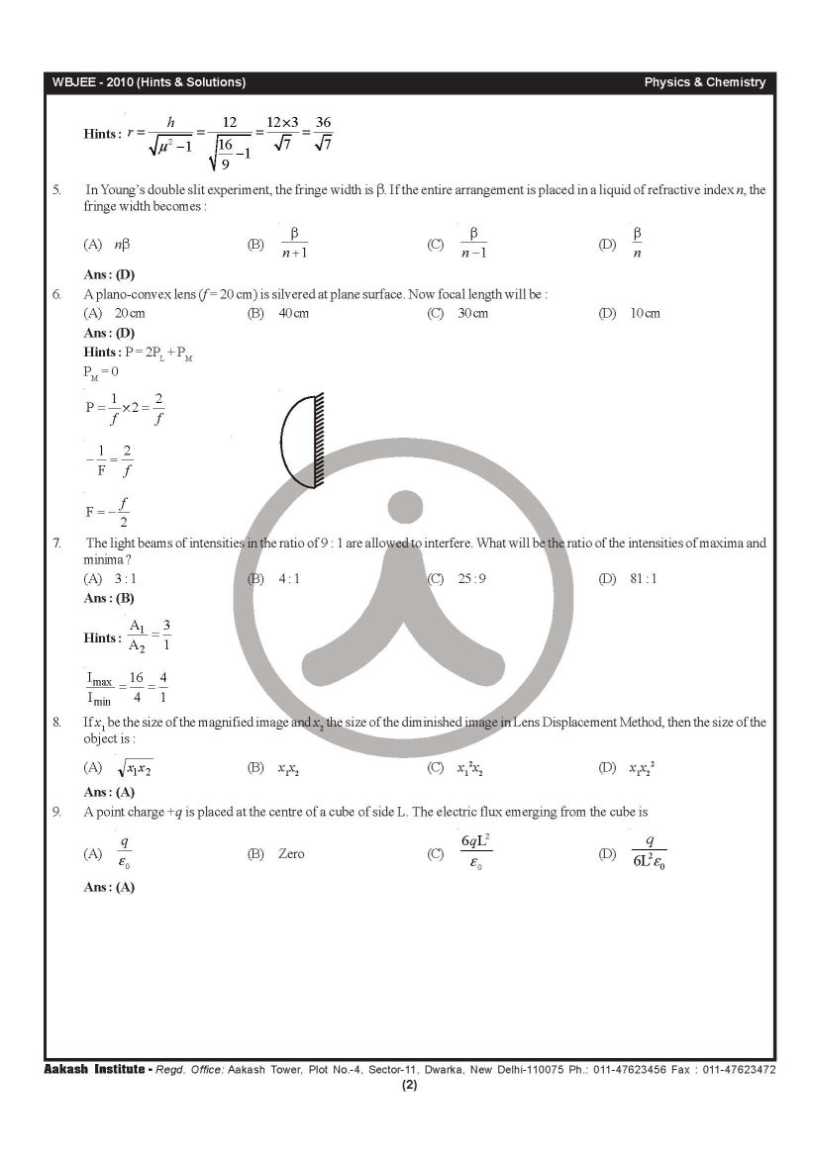 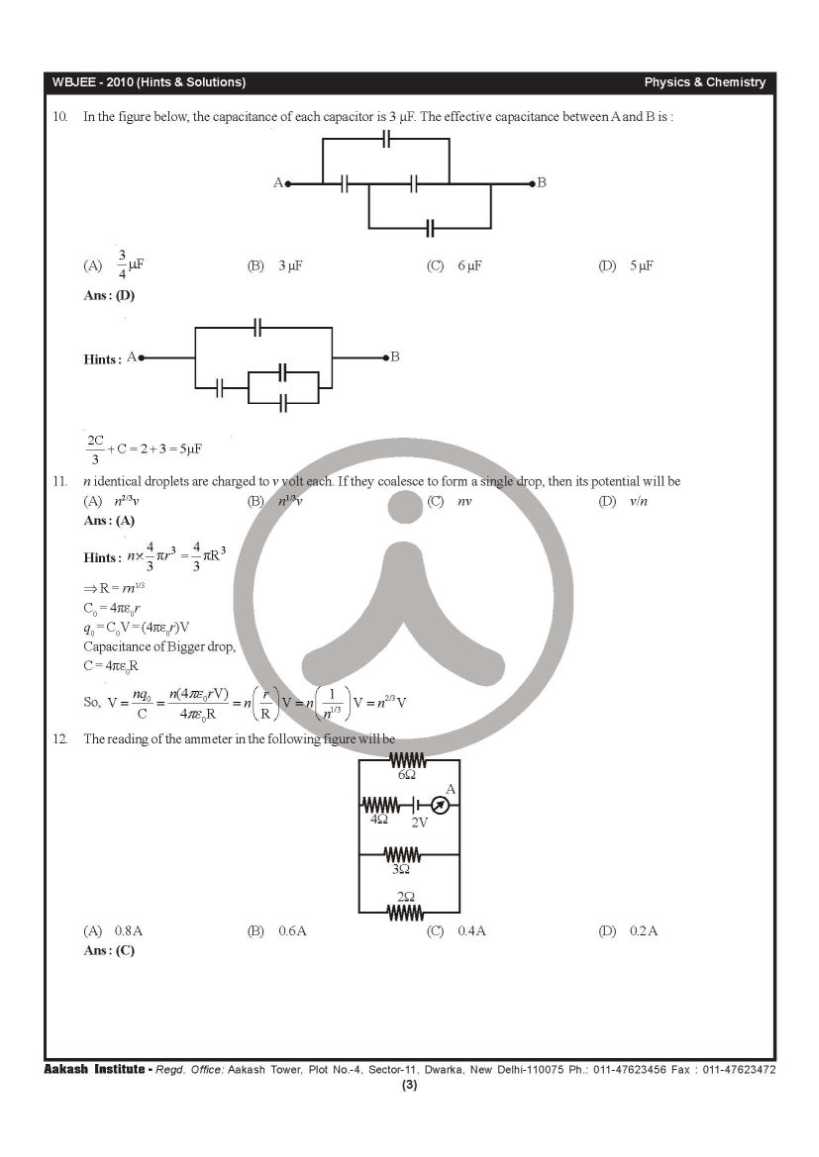 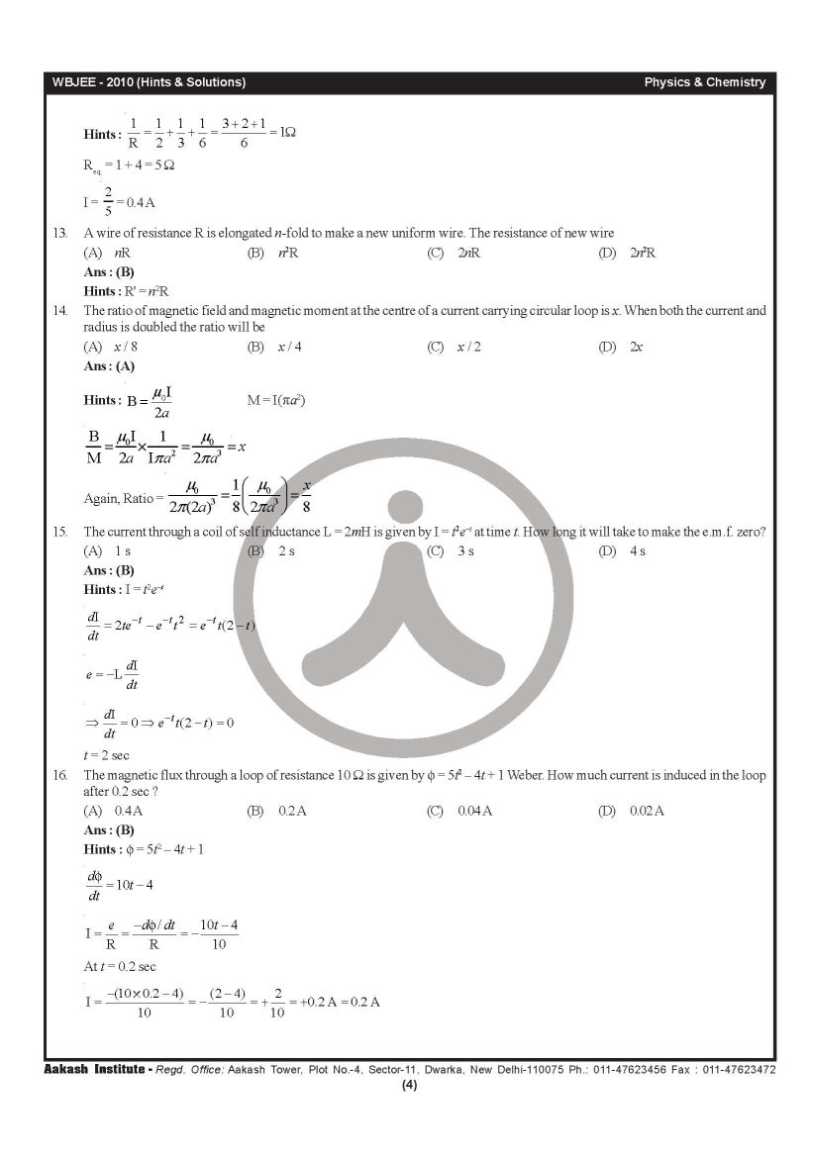 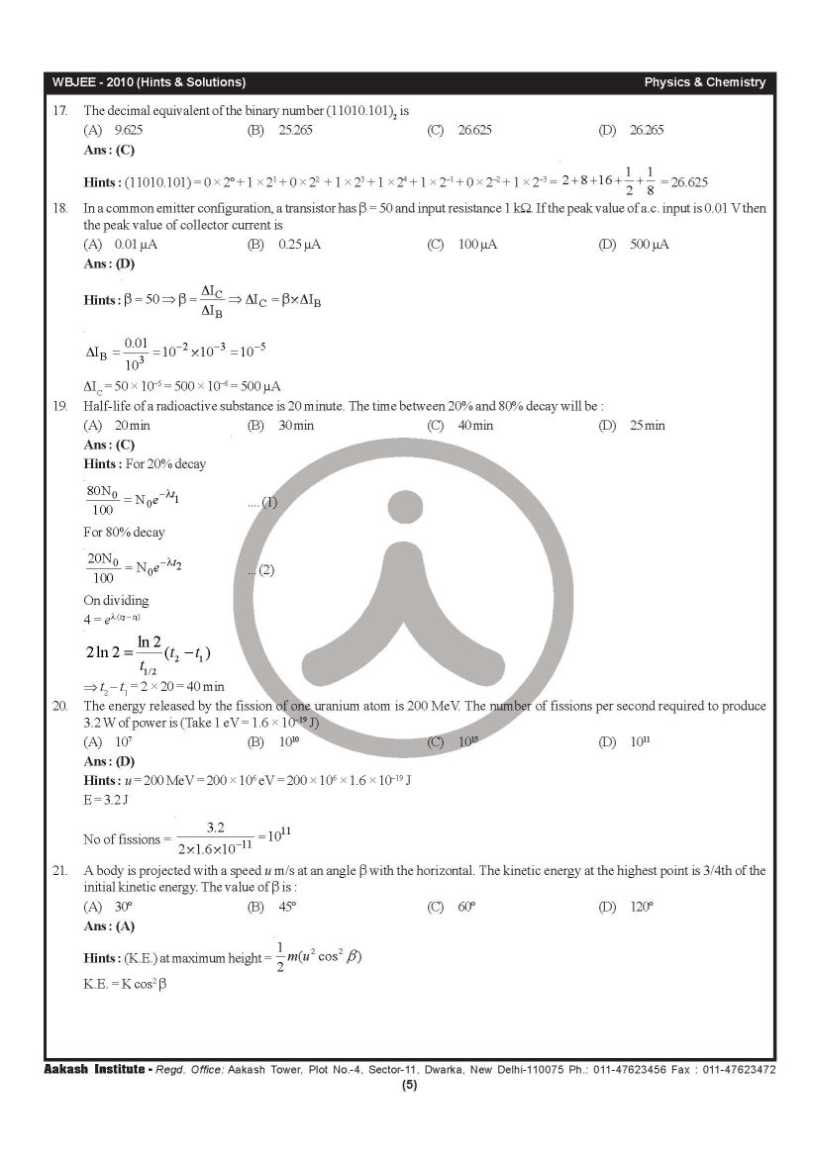 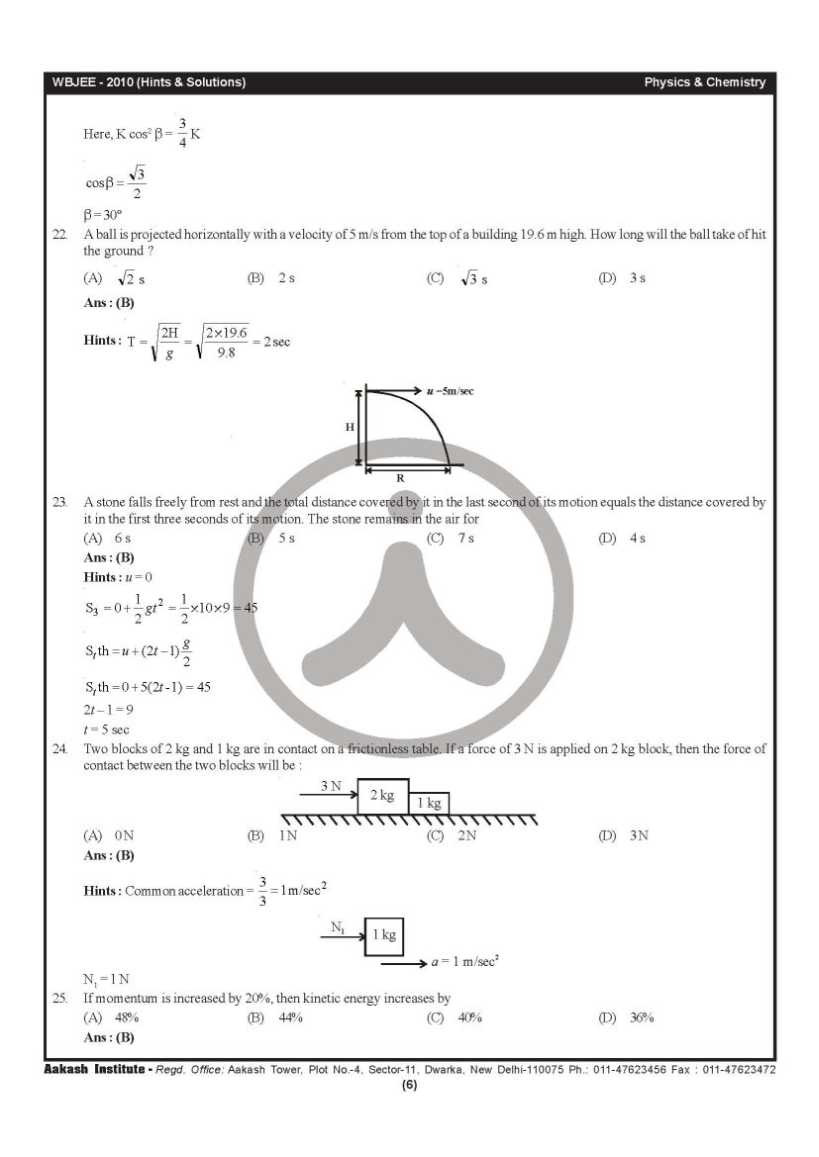 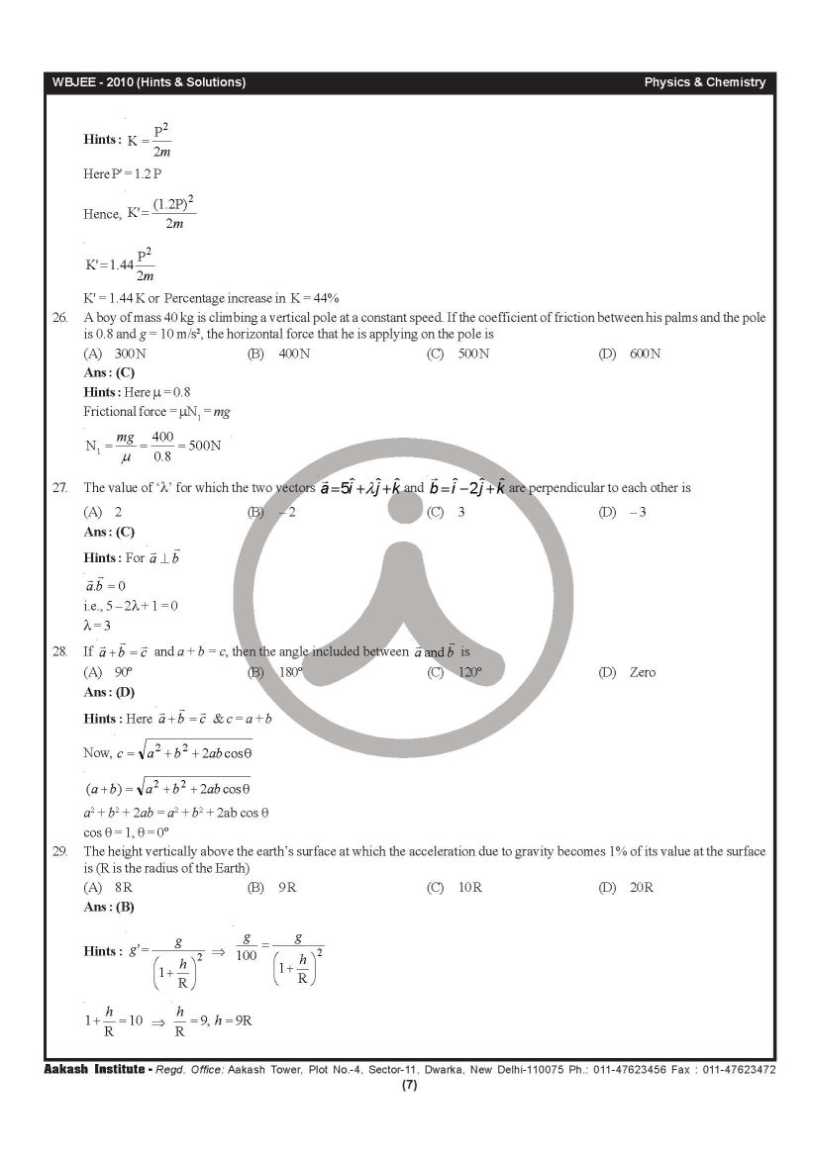 Contact Details: The West Bengal Joint Entrance Examinations Board AQ Block, Sector V, Salt Lake City, Kolkata, West Bengal 700091 India Map Location: Last edited by Aakashd; October 15th, 2019 at 12:42 PM. |
|
#3
| ||||
| ||||
|
Yes sure, here I am providing you the WBJEE old Question papers. There is objective type of the questions available. A reducing agent A. loses electrons and is reduced. B. gains electrons and is reduced. C. loses electrons and is oxidized. D. gains electrons and is oxidized. The cathode is slowly raised out of the melt by an electrical device during the electrolytic extraction of: A. sodium B. magnesium C. calcium D. aluminium Deoxyribose is: A. an aldopentose with four hydroxyl groups B. a ketopentose with four hydroxyl groups C. an aldopentose lacking hydroxyl group at carbon-3 D. an aldopentose lacking hydroxyl group at carbon-2     
__________________ Answered By StudyChaCha Member Last edited by Aakashd; July 30th, 2018 at 10:10 AM. |
|
#5
| ||||
| ||||
|
West Bengal Joint Entrance Exam Model Papers provide much help in preparation in the Examination. Some of the questions are given below. Sample Questions: Which one of the following is the correct statement? i. Chlorides of both beryllium and aluminium have bridged chloride structures in solid phase. ii. B2H6.2NH3 is known as 'inorganic benzene'. iii. Boric acid is a protonic acid. iv. Beryllium exhibits coordination number of six. The pKa of a weak acid, HA is 4.80. The pKb of a weak base, BOH, is 4.78. The pH of an aqueous solution of the corresponding salt, BA, will be i. 7.01 ii. 9.22 iii. 9.58 iv. 4.79 Amount of oxalic acid present in a solution can be determined by its titration with KMnO4solution in the presence of H2SO4. The titration gives unsatisfactory result when carried out in the presence of HCl, because HCl i. reduces permanganate to Mn2+. ii. oxidises oxalic acid to carbon dioxide and water. iii. gets oxidised by oxalic acid to chlorine. iv. furnishes H+ ions in addition to those from oxalic acid. Among the following substituted silanes the one which will give rise to cross linked silicone polymer on hydrolysis is i. R2SiCl2 ii. R3SiCl iii. R4Si iv. RSiCl3 Which of the following factors is of no significance for roasting sulphide ores to the oxides and not subjecting the sulphide ores to carbon reduction directly? i. Metal sulphides are less stable than the corresponding oxides. ii. CO2is more volatile than CS2. iii. Metal sulphides are thermodynamically more stable than CS2. iv. CO2is thermodynamically more stable than CS2.    Here I am attaching the Sample Question Papers of WBJEE Examination:
__________________ Answered By StudyChaCha Member Last edited by Aakashd; July 30th, 2018 at 10:11 AM. |
|
#8
| |||
| |||
|
This is the WBJEE Mathematics Previous years question paper: Eleven apples are distributed among a girl and a boy. Then which one of the following statements is true ? (A) At least one of them will receive 7 apples (B) The girl receives at least 4 apples or the boy receives at least 9 apples (C) The girl receives at least 5 apples or the boy receives at least 8 apples (D) The girl receives at least 4 apples or the boy receives at least 8 apples Ans : () If a, b, c are in A.P., then the straight line ax + 2by + c = 0 will always pass through a fixed point whose co-ordinates are (A) (1, –1) (B) (–1, 1) (C) (1, –2) (D) (–2, 1) Ans : (A) In the set of all 3×3 real matrices a relation is defined as follows. A matrix A is related to a matrix B if and only if there is a non-singular 3×3 matrix P such that B = P–1AP. This relation is (A) Reflexive, Symmetric but not Transitive (B) Reflexive, Transitive but not Symmetric (C) Symmetric, Transitive but not Reflexive (D) an Equivalence relation Ans : (D) For any two real numbers a and b, we define a R b if and only if sin2 a + cos2b = 1. The relation R is (A) Reflexive but not Symmetric (B) Symmetric but not transitive (C) Transitive but not Reflexive (D) an Equivalence relation Ans : (D)
__________________ Answered By StudyChaCha Member |
|
#9
| |||
| |||
|
This is the WBJEE last year’s papers with solution of physics and chemistry subject: 41. Which one of the following characteristics belongs to an electrophile? A. It is any species having electron deficiency which reacts at an electron rich C-centre B. It is any species having electron enrichment, that reacts at an electron deficient C-centre C. It is cationic in nature D. It is anionic in nature 42. Which one of the following methods is used to prepare Me3COEt with a good yield? A. Mixing EtONa with Me3CCl B. Mixing Me3CONa with EtCl C. Heating a mixture of (1:1) EtOH and Me3COH in presence of conc. H2SO4 D. Treatment of Me3COH with EtMgI 43. 58.5 gm of NaCl and 180gm of glucose were separately dissolved in 1000ml of water. Identify the correct statement regarding the elevation of boiling point (b.p.) of the resulting solutions. A. NaCl solution will show higher elevation of b.p. B. Glucose solution will show higher elevation of b.p. C. Both the solution will show equal elevation of b.p. D. The b.p. elevation will be shown by neither of the solutions  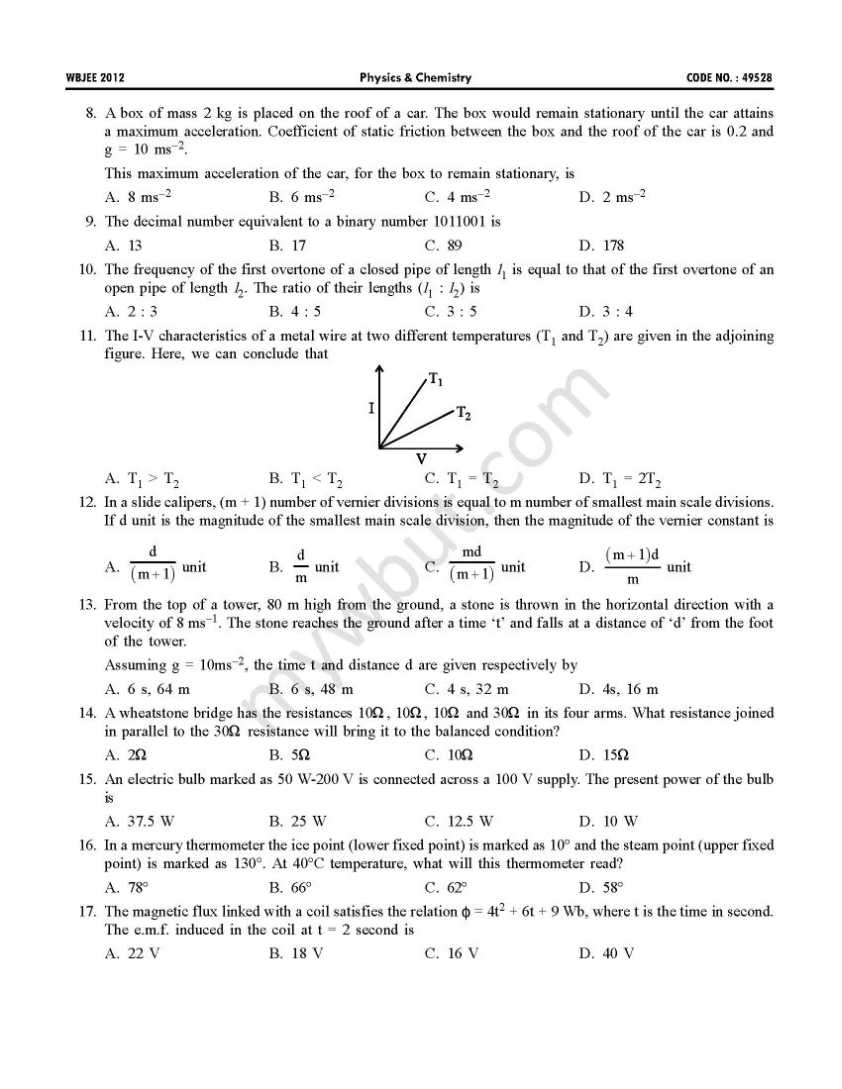       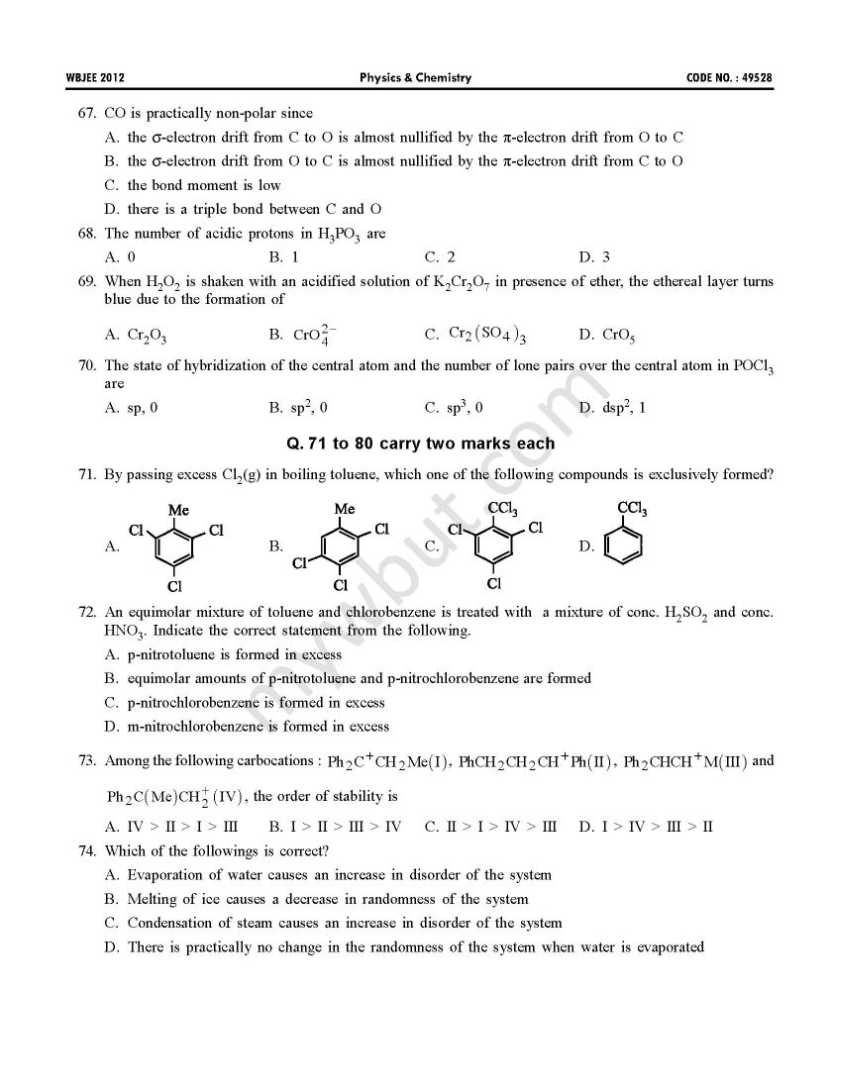  Rests of the questions are in the attachment, please click on it………….
__________________ Answered By StudyChaCha Member Last edited by Aakashd; July 30th, 2018 at 10:11 AM. |