|
#1
| |||
| |||
|
Please provide the model question papers of the NTSE Examination?
|
|
#2
| |||
| |||
|
The National Talent Search Examination (NTSE) is a national level scholarship program in India to Identify and Nurture talented students. The NTSE is divided into two broad categories Mental Ability Test (MAT), and Scholastic Aptitude Test (SAT) (with questions on history, civics, geography, rarely economics, maths, physics, chemistry and biology.) Sample Questions: Find the next number in the sequence – 9: 25: 49 36 81 64 100 Find the missing one from the 4 alternatives – LLMO: MMNO :: AABD : ? BBCE BBCD AABD ABBC If / means X, X means –, + means x and – means / then 2 + 8 X 16 – 4 / 2 = ? 4 8 10 12 Which of the following pairs is correctly matched ILO – London ICJ – Hague UNESCO – Washington WHO – Paris Arrange the following events in chronological order – (A) Creation of Bangladesh, (B) Tashkant Declaration, (C) Simla Agreement, (D) Lahore Declaration A C D B B A C D D B A C A B C D In some countries, it compulsory to install a smoke detector. If you have to install a smoke detector in a room, where will you install it? Near the window, a few feet from the room Near an electric switch board, 4 feet from the floor Near or on the ceiling Near the floor Graphite is very soft as compared to other substances because Carbon atoms are arranged in hexagonal structure Carbon atoms are arranged in such a way that they form flat layers Linkage between atoms within a layer of graphite are weak Linkage between atoms of two layers are weak In which of the following cases, a triangle ABC with base BC given can be constructed? B and C are acute angles B and C are right angles B and C are obtuse angles B is an obtuse angle and C is a right angle Q1. If ethanol reacts with oxygen it produces (a) Acetic Acid (b) Hydrocloric Acid (c) Sulphuric Acid (d) Sulphur dioxide Ans. (a) O H COOH CH O OH H C O Cr K 2 3 7 2 5 2 2 2 → Acetic Acid Q2. Fill the question mark in following reaction CH3COONa + NaOH/CaO →∆? + Na2Co3 (a) 2 C2H6 (b) CH4 (c) C2H4 (d) None of these Ans. When Sodium acetate react with sodalime, methane is produced Q3. A burner consumes one gram of LPG in 11 sec. What is the power of consumption of burner in KW if Cv of LPG is 55 kJ/g. (a) 5 kW (b) 10 kW (c) 5.5 kW (d) None of these Ans. (a) Power = E / T = 55 / 11 = 5 kW, Time (T) = 1 sec. Q4. The ideal gas equation is (a) P1T1 / V1 = P2V2 / T2 (b) P1V1 / T1 = P2V2 / T2 (c) P1V1T1 = P2V2T2 (d) P1V1 / T2 = P2V2 / T1 Ans. (b) Q5. Dalton’s Law of partial pressure is obeyed in which one of the following pair of gases (a) Oxygen & Nitrogen (b) Nitrogen & Hydrogen (c) Hydrogen & Argon (d) Hydrogen & oxygen Ans. (c) Q6. The molecular formula of a compound in (CO)x and its vapour density is 70. Then the possible value of x is (a) 2 (b) 10 (c) 5 (d) 9 Ans. (c) Q7. Number of groups present in the long form of the periodic table are (a) 16 (b) 8 (c) 2 (d) 18 Ans. (a) Q8. Which of the following is a neutral oxide (a) NO (b) NO2 (c) N2O5 (d) CO2 Ans. (a) Q9. Molecular weight of a substance is equivalent to (a) Sum of atomic wt. of each element present in the given substance (b) Sum of At.wt of each element with their respective number present in each compound (c) Sum of equivalent wt. of each element present in the given substance (d) None of these Ans. (b) Q10. Covalent linkages is formed by (a) Transfer of electrons (b) Mutual sharing of electrons (c) Transfer as well as mutual sharing of electrons (d) None of these Ans. (b) Q11. If an acid having construction as 0.01 N is diluted to 1000 times then the PH of that acid is ? (a) 5 (b) 2 (c) 3 (d) 10 Ans. (a) Q12. Oxygen has two isotopes O16 & O18. If the percentage of O16 is 90 then the atomic weight of oxygen will be (a) 16 (b) 16.2 (c) 16.4 (d) None of these Ans. (b) Q13. Atom that can neither gain nor lose electrons is said to be (a) an Inert (b) Atomsperic (c) Metalic (d) Non – metalic Ans. (a) Q14. When a burning splinter is brought near the gas jar containing hydrogen gas a poping sound is observed. It is due to (a) exothermic (b) endothermic (c) exothermic & endothermic (d) None of these Ans. (a) Q15. In which of the following preparation Hydrogen is not used? (a) preparation of Ammonia (NH3) (b) Hydrogenetion of oil (c) Synthesis of water gas (d) all of these Ans. (d) Q16. Deacon’s process is used for the manufacturing of (a) Bleaching powder (b) Sulphuric acid (c) chlorine (d) Hydrochloric acid (HCL) Ans. (c) Q17. Which one of the following method is considered to be a best method for the removal of temporary hardness of water (a) Caylon’s process (b) Clark’s process (c) Vesence process (d) Permutti’s process Ans. (b) Q18. When chlorine gas is passed through NaoH, it forms (a) Sodium chloride (b) Sodium chlorate (c) Sodium hypochlorite (d) All of these Ans. (d) Q19. Skin becomes yellow in Conc. H2SO4 as (a) HNO3 acts as an oxidizing agent (b) HNO3 acts as a dehydrating agent (c) Nitro – cellulose is formed (d) The proteins are converted into xantho proteins Ans. (d) Q20. Which of the following is used as a moderator in nuclear reactor (a) Water (b) Heavy water (c) Active Hydrogen (d) Heavy Hydrogen Ans. (b) Q21. Which one of the following is known as “King of Chemicals” (a) Hydrochloric acid (b) Sulphuric acid (c) Nitric acid (d) Phosphoric acid Ans. (b) Q22. The common gas used in our refrigerator (a) maresh gas (b) producer gas (c) freon (d) water gas Ans. (c) Q23. Alum is added with muddy water to (a) Kill bactaria (b) Make filtration of milk (c) Make the sedimsitation process quick (d) None of these Ans. (c) Q24. Alloy is a homogenous mixture of (a) two or more metals (b) a metal & a non metal (c) metals as well as non metals (d) all of these Ans. (c) Q25. To protect the metal from corrosion it is some times coated with a thin layer of Al2O3 (Aluminium oxide) and the process is called (a) Electroplating (b) Electroforming (c) Aluminizing (d) None of these Ans. (b) Q26. The I.U.P.A.C. name of the compound CH2 CH2 CH (CH3)2 is (a) N – Propene (b) 3 methyl butane (c) 2 methyl butene (d) None of these Ans. (c) Q27. Fuel in Automobiles is a mixture of (a) saturated hydrocarbons (b) unsaturated hydrocarbons (c) crude oil (d) saturated & unsaturated hydrocarbons Ans. (c) Q28. In the soda fire extinguishes due to (a) formation of CO2 (b) presence of sodium bicarbonate (c) formation of water as a product (d) None of these Ans. (a) Q29. The glasses which is used for making lenses and prisms for optical instrument (a) Hard glass (b) Pyrex glass (c) Croked glass (d) Tint glass Ans. (c) Q30. Which of the following is of a thermoplastic? (a) Teflon (b) Orlon (c) Bakelite (d) Polythene Here I am attaching the model question papers of the NTSE Examination:
__________________ Answered By StudyChaCha Member |
|
#3
| |||
| |||
|
Here I am providing the list of few questions of NTSE exam Question Paper which you are looking for . Q1. One third of a number is greater then one fourth of its successor by 1, find the number (a) 15 (b) 20 (c) 5 (d) 25 Ans. Number = x, Successor = x + 1 Q2. The sum of two numbers is 24 & the sum of their reciprocal is 5 1 , find their product (a) 80 (b) 100 (c) 60 (d) 40 For more questions , here is the attachment  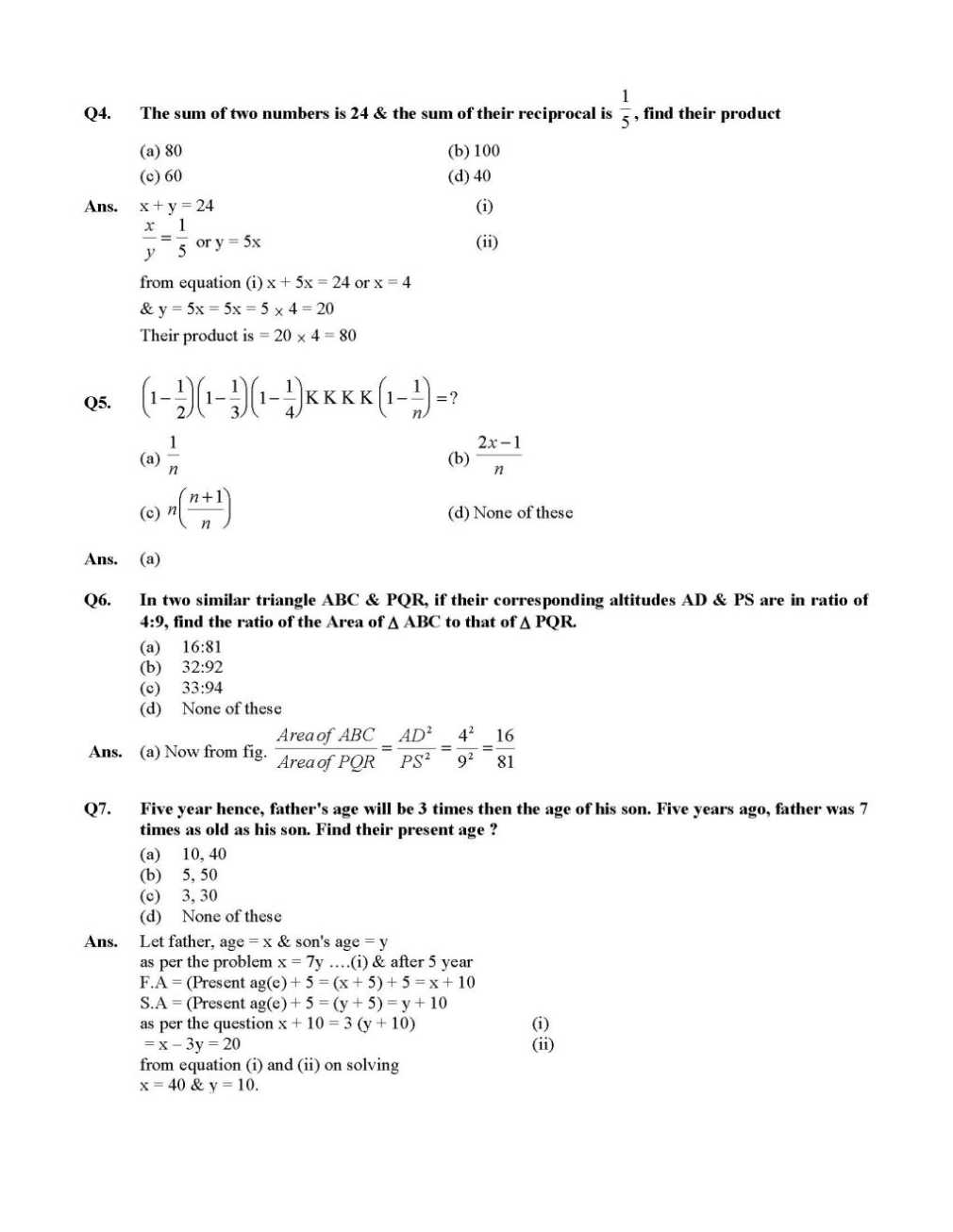 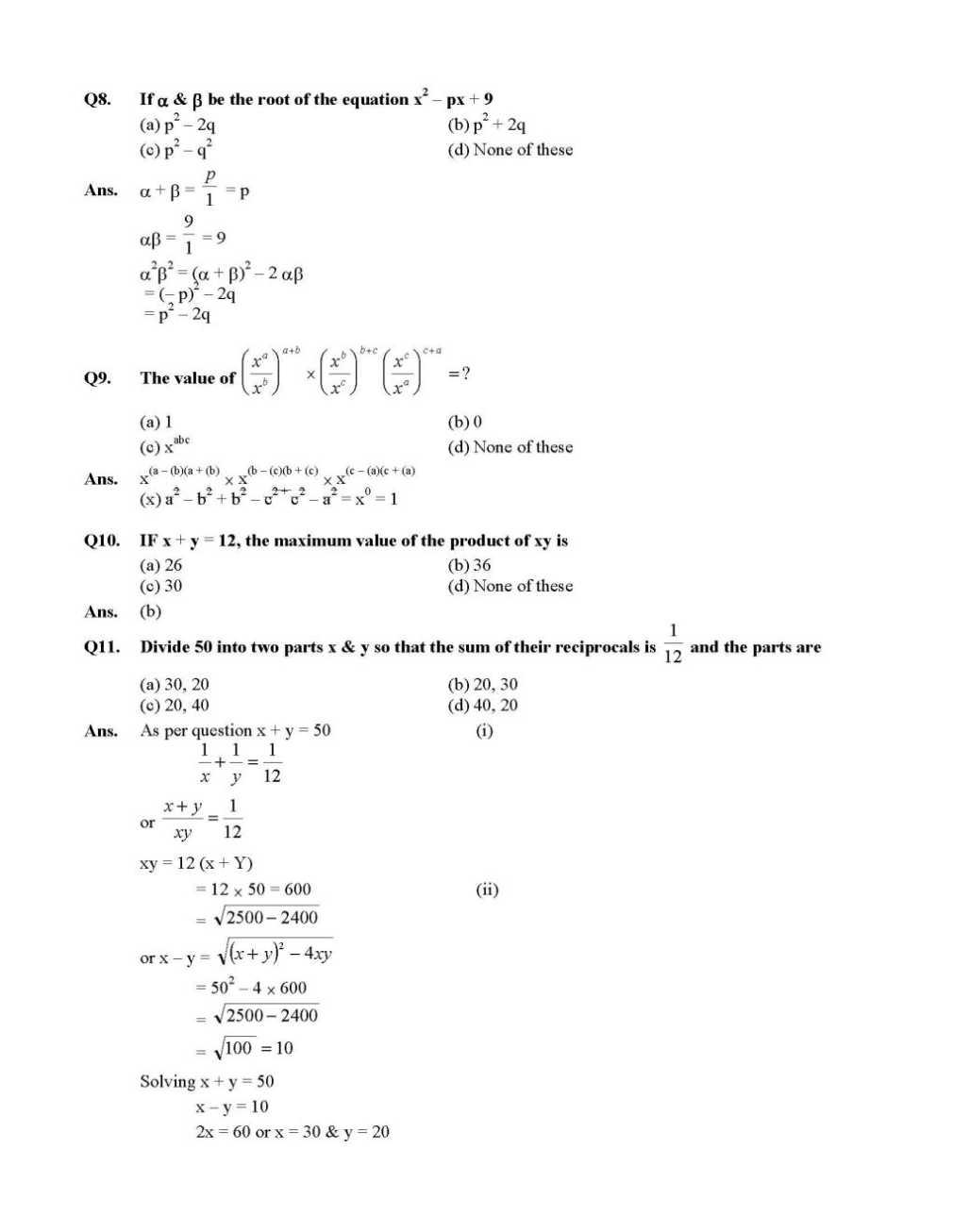  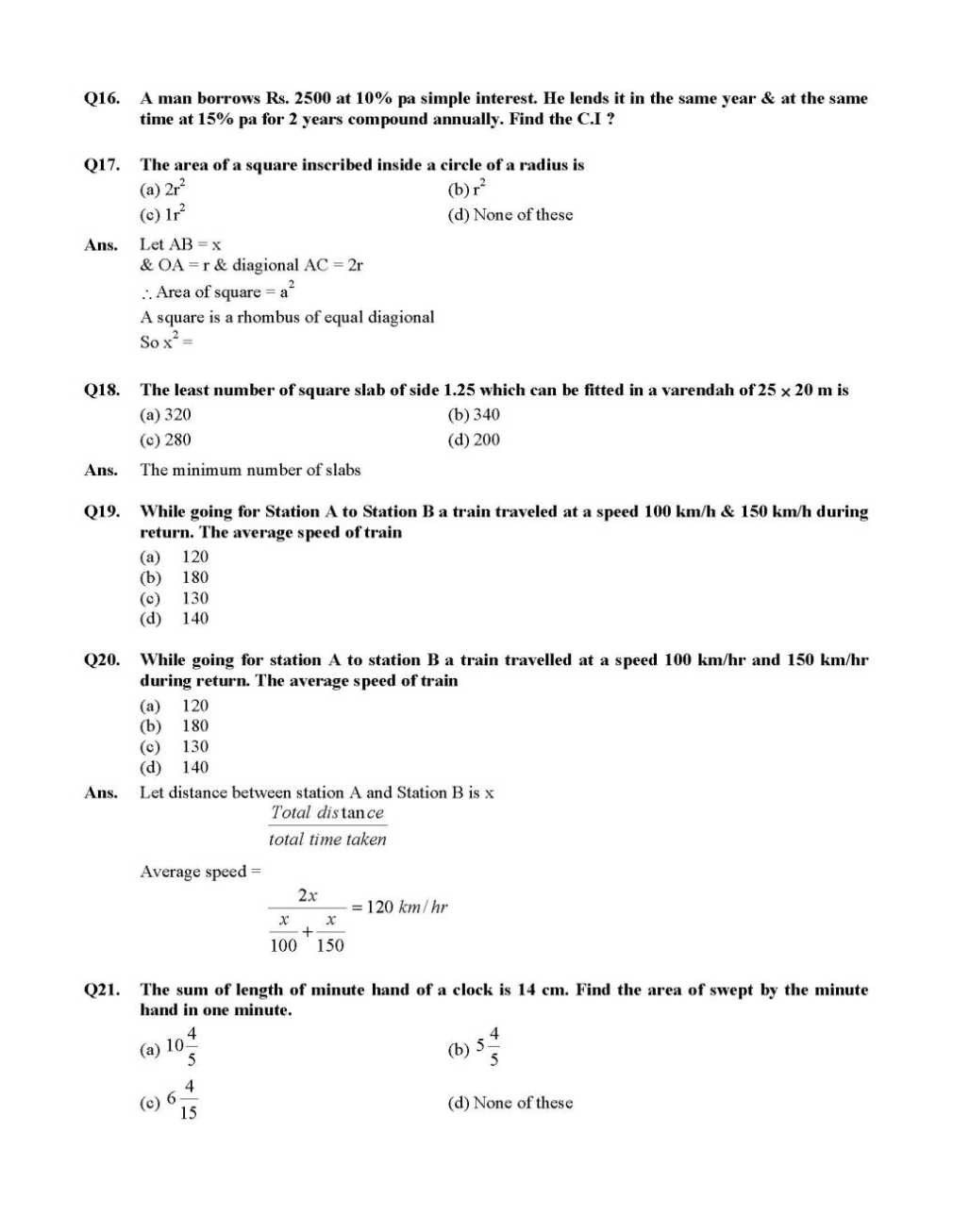
__________________ Answered By StudyChaCha Member |