|
#1
| |||
| |||
|
Will you please share the last Year Question Papers for IIT JEE Examination???
|
|
#2
| |||
| |||
|
Here I am sharing the last Year Question Papers for IIT JEE Examination Geometrical shapes of the complexes formed by the reaction of Ni2 with Cl, CN and H20, respectively, are (A) octahedral, tetrahedral and square planar (B) tetrahedral, square planar and octahedral (C) square planar, tetrahedral and octahedral (D) octahedral, square planar and octahedral Extraction of metal from the ore cassiterite involves (A)carbon reduction of an oxide ore (B) self-reduction of a suiphide ore removal of copper impurity (D) removal of iron impurity According to kinetic theory of gases (A) collisions are always elastic. (B) heavier molecules transfer more momentum to the wall of the container. (C) only a small number of molecules have very high velocity. (0) between collisions, the molecules move in straight lines with constant velocities. A police car with a siren of frequency 8 kHz is moving with uniform velocity 36 km/hr towards a tall building which reflects the sound waves. The speed of sound in air is 320 mIs. The frequency of the siren heard by the car driver is (A) 8.50 kHz (B) 8.25 kHz (C) 7.75 kHz (D) 7.50 kHz        Rest of the Questions are attached in below file which is free of cost () ()
__________________ Answered By StudyChaCha Member |
|
#4
| |||
| |||
|
For you reference, here I am giving you Last Year Papers of IIT JEE exam, please have a look….. Q. 1 Let X be the energy needed to raise the temperature of 5 moles of nitrogen held at constant pressure by one degree. Let Y be the energy needed to raise 5 moles of carbon monoxide by one degree with the pressure held constant. What is the ratio X:Y? 5:7 1:1 7:5 7:9 Answer: B Q. 2 The difference Cp - Cv is a constant. This constant is often called R, the universal gas constant. Which of the following is true given the data? For a monoatomic gas, Cp = 3/2 R For a diatomic gas, Cp = 3/2 R For a monoatomic gas, Cv = 3/2 R For a diatomic gas, Cv = 3/2 R Answer: C Q. 3 How much energy would be required to heat two moles of methane by one degree if the gas is kept at constant volume? 6.5 calories 8.5 calories 11 calories 13 calories Answer: D Q. 4 Which of the following is a possible explanation for the fact that Cp is always greater than Cv? Some of the energy is used to expand the container in order to maintain constant pressure. A rigid container does not conduct heat as well as one that can change shape. There are generally more moles of gas when the pressure is kept constant than when the volume is kept constant. There are generally fewer moles of gas when the pressure is kept constant than when the volume is kep constant. Answer: A Q. 5 A certain amount of energy, X, is sufficient to raise the temperature of 60 moles of argon by T degrees when the pressure is constant. How many moles of argon can be raised by T degrees with the same amount of energy X, if the volume is held constant? 30 50 75 100 Answer: D Last Year Papers for IIT JEE   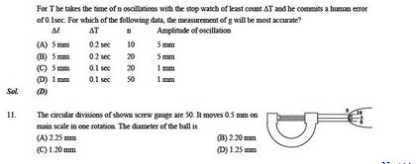    
__________________ Answered By StudyChaCha Member |