|
#1
| |||
| |||
|
Hello sir I am here as I want to get the sample/Previous year question paper of CSIR UGC NET of Chemical Science so will you please provide me the paper?
|
|
#2
| |||
| |||
|
Hey!!!! As per your demand here I am providing you Previous year question paper of CSIR UGC NET of Chemical Science Question paper of CSIR UGC NET of Chemical Science   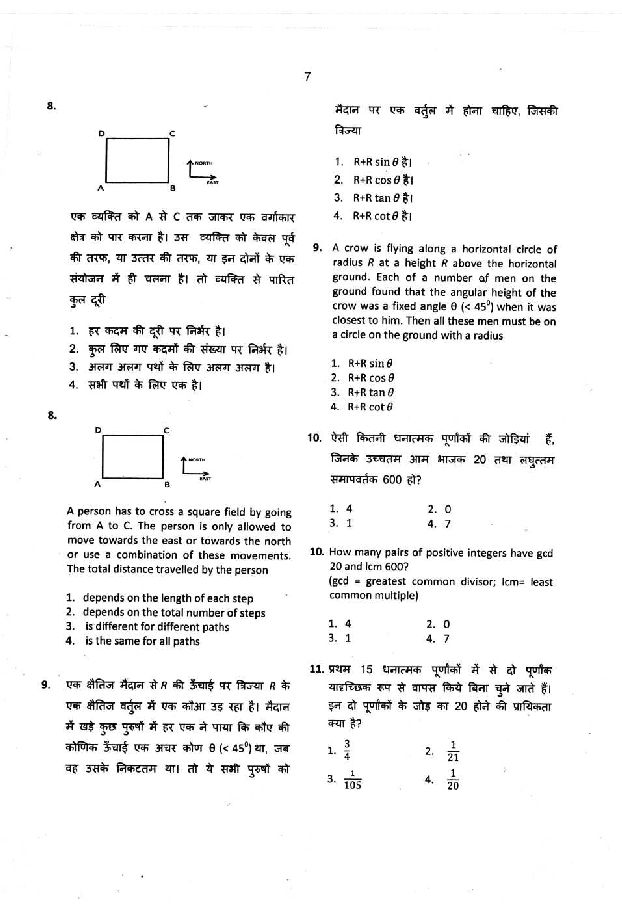 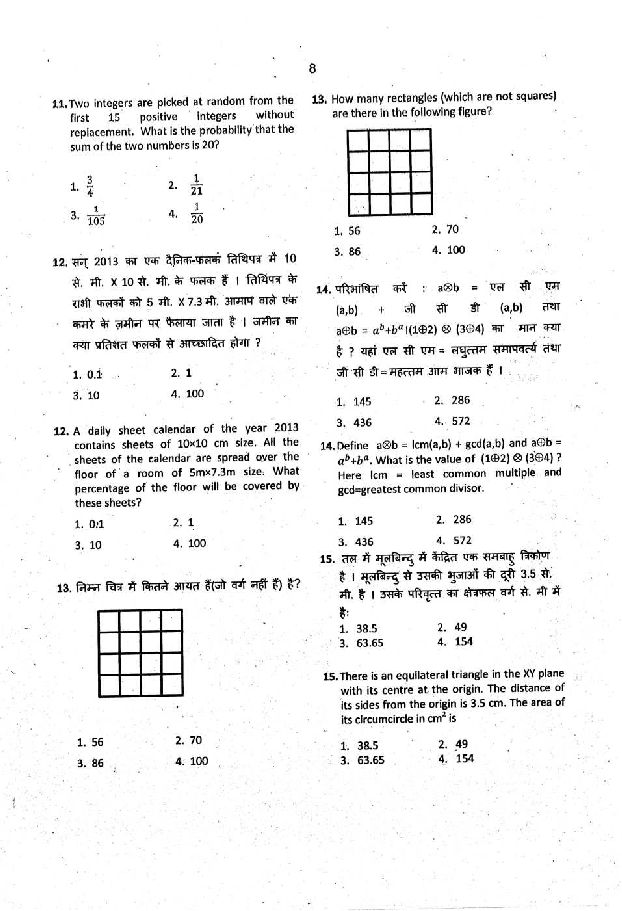 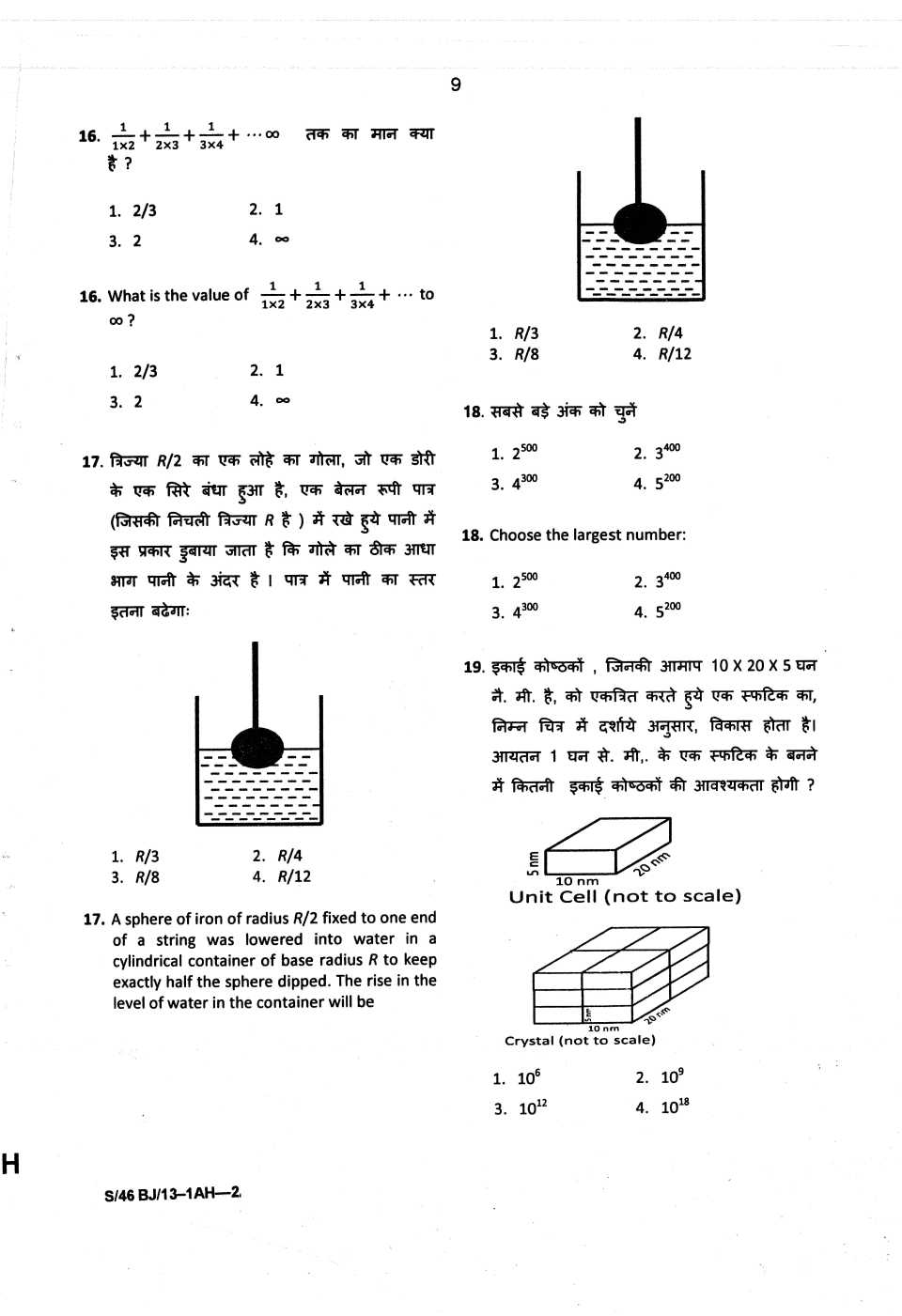 Syllabus of CSIR UGC NET of Chemical Science Inorganic Chemistry 1. Chemical periodicity 2. Structure and bonding in homo- and heteronuclear molecules, including shapes of molecules (VSEPR Theory). 3. Concepts of acids and bases, Hard-Soft acid base concept, Non-aqueous solvents. 4. Main group elements and their compounds: Allotropy, synthesis, structure and bonding, industrial importance of the compounds . 5. Transition elements and coordination compounds: structure, bonding theories, spectral and magnetic properties, reaction mechanisms. 6. Inner transition elements: spectral and magnetic properties, redox chemistry, analytical applications. 7. Organometallic compounds: synthesis, bonding and structure, and reactivity. Organometallics in homogeneous catalysis. 8. Cages and metal clusters. 9. Analytical chemistry- separation, spectroscopic, electro- and thermoanalytical methods. 10. Bioinorganic chemistry: photosystems, porphyrins, metalloenzymes, oxygen transport, electron- transfer reactions; nitrogen fixation, metal complexes in medicine. 11. Characterisation of inorganic compounds by IR, Raman, NMR, EPR, Mössbauer, UV-vis, NQR, MS, electron spectroscopy and microscopic techniques. 12. Nuclear chemistry: nuclear reactions, fission and fusion, radio-analytical techniques and activation analysis. Physical Chemistry: 1. Basic principles of quantum mechanics: Postulates; operator algebra; exactly- solvable systems: particle-in-a-box, harmonic oscillator and the hydrogen atom, including shapes of atomic orbitals; orbital and spin angular momenta; tunneling. 2. Approximate methods of quantum mechanics: Variational principle; perturbation theory up to second order in energy; applications. 3. Atomic structure and spectroscopy; term symbols; many-electron systems and antisymmetry principle. 4. Chemical bonding in diatomics; elementary concepts of MO and VB theories; Huckel theory for conjugated π-electron systems. 5. Chemical applications of group theory; symmetry elements; point groups; character tables; selection rules. 6. Molecular spectroscopy: Rotational and vibrational spectra of diatomic molecules; electronic spectra; IR and Raman activities – selection rules; basic principles of magnetic resonance. 7. Chemical thermodynamics: Laws, state and path functions and their applications; thermodynamic description of various types of processes; Maxwell’s relations; spontaneity and equilibria; temperature and pressure dependence of thermodynamic quantities; Le Chatelier principle; elementary description of phase transitions; phase equilibria and phase rule; thermodynamics of ideal and non-ideal gases, and solutions. 8. Statistical thermodynamics: Boltzmann distribution; kinetic theory of gases; partition functions and their relation to thermodynamic quantities – calculations for model systems. 9. Electrochemistry: Nernst equation, redox systems, electrochemical cells; Debye-Huckel theory; electrolytic conductance – Kohlrausch’s law and its applications; ionic equilibria; conductometric and potentiometric titrations. 10. Chemical kinetics: Empirical rate laws and temperature dependence; complex reactions; steady state approximation; determination of reaction mechanisms; collision and transition state theories of rate constants; unimolecular reactions; enzyme kinetics; salt effects; homogeneous catalysis; photochemical reactions. 11. Colloids and surfaces: Stability and properties of colloids; isotherms and surface area; heterogeneous catalysis. 12. Solid state: Crystal structures; Bragg’s law and applications; band structure of solids. 13. Polymer chemistry: Molar masses; kinetics of polymerization. 14. Data analysis: Mean and standard deviation; absolute and relative errors; linear regression; covariance and correlation coefficient. Organic Chemistry 1. IUPAC nomenclature of organic molecules including regio- and stereoisomers. 2. Principles of stereochemistry: Configurational and conformational isomerism in acyclic and cyclic compounds; stereogenicity, stereoselectivity, enantioselectivity, diastereoselectivity and asymmetric induction. 3. Aromaticity: Benzenoid and non-benzenoid compounds – generation and reactions. 4. Organic reactive intermediates: Generation, stability and reactivity of carbocations, carbanions, free radicals, carbenes, benzynes and nitrenes. 5. Organic reaction mechanisms involving addition, elimination and substitution reactions with electrophilic, nucleophilic or radical species. Determination of reaction pathways. 6. Common named reactions and rearrangements – applications in organic synthesis. 7. Organic transformations and reagents: Functional group interconversion including oxidations and reductions; common catalysts and reagents (organic, inorganic, organometallic and enzymatic). Chemo, regio and stereoselective transformations. 8. Concepts in organic synthesis: Retrosynthesis, disconnection, synthons, linear and convergent synthesis, umpolung of reactivity and protecting groups. 9. Asymmetric synthesis: Chiral auxiliaries, methods of asymmetric induction – substrate, reagent and catalyst controlled reactions; determination of enantiomeric and diastereomeric excess; enantio-discrimination. Resolution – optical and kinetic. 10. Pericyclic reactions – electrocyclisation, cycloaddition, sigmatropic rearrangements and other related concerted reactions. Principles and applications of photochemical reactions in organic chemistry. 11. Synthesis and reactivity of common heterocyclic compounds containing one or two heteroatoms (O, N, S). 12. Chemistry of natural products: Carbohydrates, proteins and peptides, fatty acids, nucleic acids, terpenes, steroids and alkaloids. Biogenesis of terpenoids and alkaloids. 13. Structure determination of organic compounds by IR, UV-Vis, 1H & 13C NMR and Mass spectroscopic techniques. Interdisciplinary topics 1. Chemistry in nanoscience and technology. 2. Catalysis and green chemistry. 3. Medicinal chemistry. 4. Supramolecular chemistry. 5. Environmental chemistry.
__________________ Answered By StudyChaCha Member |