|
#1
| |||
| |||
|
Hi buddy I want to do my graduation in Presidency University Dept Of Chemistry,so can you here provide me its UG program syllabus here ???
|
|
#2
| |||
| |||
|
Presidency University, Kolkata was established in 1817 Department of Chemistry & Biochemistry at Presidency University (erstwhile Presidency College) dates back to mid-nineteenth century, it offers three-year undergraduate courses both in Chemistry and Biochemistry (intake capacities: Chemistry – 42; Biochemistry – 10), and two-year postgraduate courses in Chemistry (intake capacity: 30). FIRST SEMESTER Course No. CHEM0101 (FM=50; C=4) Group A: Organic Chemistry (M=16) General Introduction: Functional group based classification and nomenclature. Sources / origin of different compounds. Molecular formula and IHD / DBE. Bonding, Structure and Physical Properties of Organic Molecules: Valence bond theory: Concept of hybridisation, resonance (including hyperconjugation), inductive effect, steric effect, steric inhibition of resonance. Orbital pictures of bonding (sp3, Sp2, sp: C-C, C-N & C-O system). MO theory: Sketch and energy levels of MOs of i) acyclic π orbital system (C=C, conjugated diene and allyl systems) ii) cyclic π orbital system (neutral system: [4], [6] annulenes; charged system: 3, 4, 5-membered ring system); Frost diagram, Huckel's rules for aromaticity, antiaromaticity; homoaromaticity. Physical properties: Melting point, boiling point; solubility; dipole moment; acid and base strength. Group B: Inorganic & Physical Chemistry (M=34) Atomic Structure and Bonding (M=16) Why study quantum mechanics; Double slit experiment, Heisenberg's uncertainty principle, Wave-Particle duality, idea of de Broglie matter waves. Schrodinger equation, Hydrogenic wavefunctions, Quantum numbers, introduction to the concept of atomic orbitals; shapes, radial and angular probability diagrams of s, p and d orbitals (qualitative idea). Many electron atoms and ions: Pauli's exclusion principle, Hund's rule, exchange energy, Aufbau principle and its limitation. Term symbols of atoms and ions for atomic numbers <30. Linear combination of atomic orbitals, hybrid orbitals; orbital picture of bonding, Radioactivity (M=9) Nuclear stability and nuclear binding energy. Nuclear forces: meson exchange theory. Nuclear models (elementary idea): Concept of nuclear quantum number, magic numbers. Nuclear Reactions: Artificial radioactivity, transmutation of elements, fission, fusion and spallation. Nuclear energy and power generation. Separation and uses of isotopes. Basic instrumentation, measurement of radioactivity, principles of isotope dilution analysis, neutron activation analysis Radio chemical methods: principles of determination of age of rocks and minerals, radio carbon dating, hazards of radiation and safety measures Presidency University Dept Of Chemistry UG course syllabus 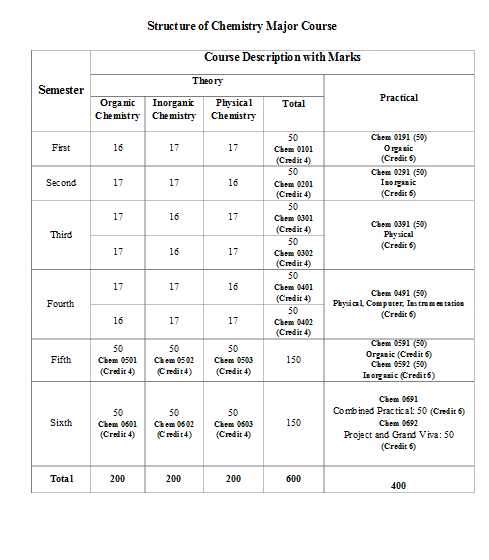 
__________________ Answered By StudyChaCha Member |