|
#1
| |||
| |||
|
My name is Akansha Babel and studying at IIT Mumbai. I want to get few previous exam papers so mention me any site to get this.
|
|
#2
| |||
| |||
|
You are studying at IIT Mumbai so you are looking for the pervious year paper so I am have listed here some sample questions and also uploaded here a PDF file for more questions. Q. 1 Let X be the energy needed to raise the temperature of 5 moles of nitrogen held at constant pressure by one degree. Let Y be the energy needed to raise 5 moles of carbon monoxide by one degree with the pressure held constant. What is the ratio X:Y? 5:7 1:1 7:5 7:9 Answer: B Discription: There is no need to do any calculations here. If you put X on top of Y, you will see that it reduces to the ratio of Cp for nitrogen over Cp for carbon monoxide. And since both gases have the same Cp, the ratio is simply 1:1. Q. 2 The difference Cp - Cv is a constant. This constant is often called R, the universal gas constant. Which of the following is true given the data? For a monoatomic gas, Cp = 3/2 R For a diatomic gas, Cp = 3/2 R For a monoatomic gas, Cv = 3/2 R For a diatomic gas, Cv = 3/2 R Answer: C Discription: Helium and argon are monoatomic gases, and we can see that Cv = 3/2 *R = (3/2)*2 = 3. Q. 3 How much energy would be required to heat two moles of methane by one degree if the gas is kept at constant volume? 6.5 calories 8.5 calories 11 calories 13 calories Answer: D Description: The definition of Cv is the amount of energy required to heat one mole of a gas by one degree. Therefore, to heat two moles of methane by one degree will require 2*6.5 = 13 calories. Q. 4 Which of the following is a possible explanation for the fact that Cp is always greater than Cv? Some of the energy is used to expand the container in order to maintain constant pressure. A rigid container does not conduct heat as well as one that can change shape. There are generally more moles of gas when the pressure is kept constant than when the volume is kept constant. There are generally fewer moles of gas when the pressure is kept constant than when the volume is kep constant. Answer: A Discription: Because Cp is always greater than Cv we know that it takes more energy to increase a given amount of gas when the pressure is held constant. It is reasonable that the extra energy is used to increase the volume of the container. Q. 5 A certain amount of energy, X, is sufficient to raise the temperature of 60 moles of argon by T degrees when the pressure is constant. How many moles of argon can be raised by T degrees with the same amount of energy X, if the volume is held constant? 30 50 75 100 Answer: D Discussion: You can use the two equations: You are given that X = 60*5*T. Let n be the number of moles that can be heated to temperature T with X calories of energy. Then X = n*3*T, and for this equation to be consistent with the previous one, it must be that n = 100.
__________________ Answered By StudyChaCha Member |
|
#3
| |||
| |||
|
As you want I am here providing you sample paper of the IIT Examination. sample paper: 21. Which ordering of compounds is according to the decreasing order of the oxidation state of nitrogen? (A) HNO3, NO, NH4Cl, N2 (B) HNO3, NO, N2, NH4Cl (C) HNO3, NH4Cl, NO, N2 (D) NO, HNO3, NH4Cl, N2 Ans. (B) Sol. HNO3 = + 5 NO = +2 NH4Cl = N2 = 0 So correct order will be HNO3, NO,N2, NH4Cl. 28. As per IUPAC nomenclature, the name of the complex [Co(H2O)4(NH3)2]Cl3 is : (A) Tetraaquadiaminecobalt (III) chloride (B) Tetraaquadiamminecobalt (III) chloride (C) Diaminetetraaquacoblat (III) chloride (D) Diamminetetraaquacobalt (III) chloride Ans. (D) Sol. [Co(H2O)4 (NH3)2 ]Cl3 = Diamminetetraaquacobalt (III) chloride. 29. The carboxyl functional group ( ˇ (A) picric acid (B) barbituric acid (C) ascorbic acid (D) aspirin Ans. (D) Sol . picric acid ; barbituric acid ascorbic acid ; aspirin 30. The colour of light absorbed by an aqueous solution of CuSO4 is : (A) organge-red (B) blue-green (C) yellow (D) violet Ans. (A) Sol. CuSO4 will be absorbing orange-red colour & hence will be of blue colour. 34. Choose the correct reason(s) for the stability of the lyophobic colloidal particles. (A) Preferential adsorption of ions on their surface from the solution. (B) Preferential adsorption of solvent on their surface from the solution. (C) Attraction between different particles having opposite charges on their surface. (D) Potential difference between the fixed layer and the diffused layer of opposite charges around the colloidal particles. Ans. (AD) Sol. (A) due to preferential adsorption of common ions (B)X (C) X (due to repulsion not due to attraction) (D) The layer of oppositely charged particles around any colloidal particles will decrease the potential energy of system as a whole. Sample Paper : IIT exam 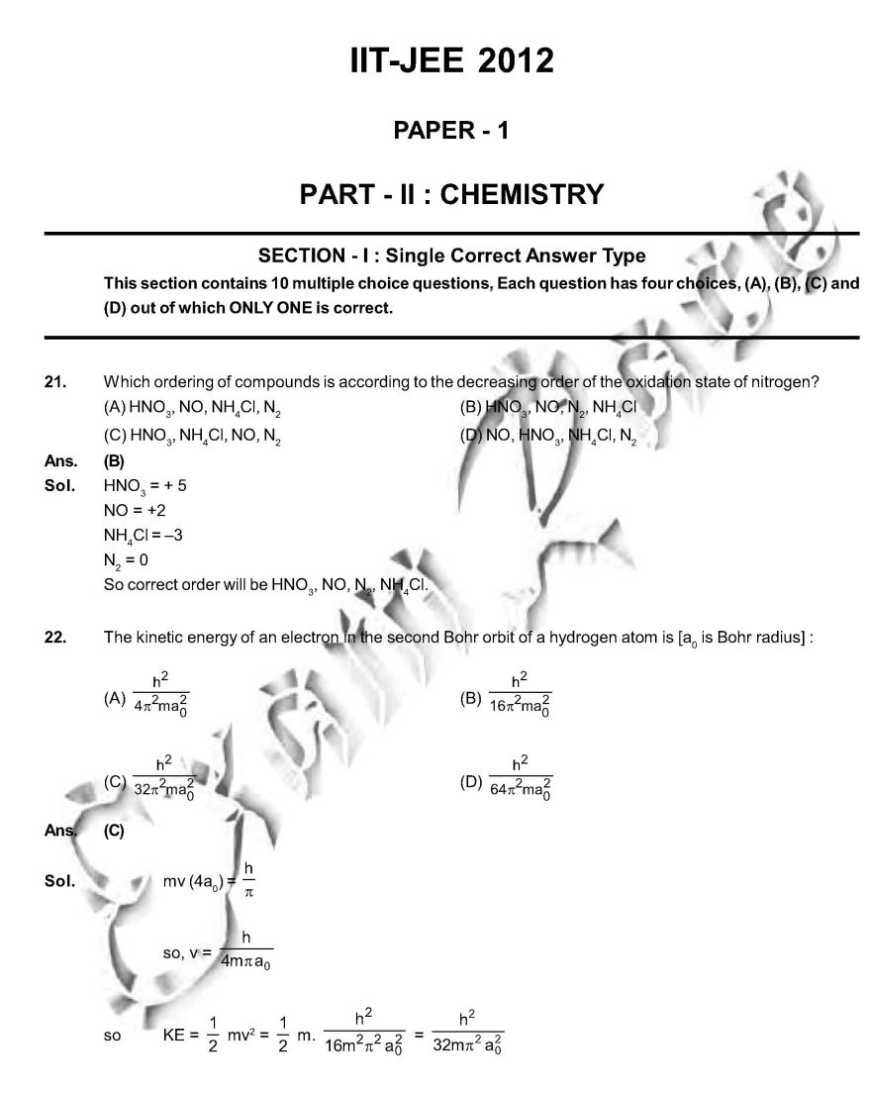 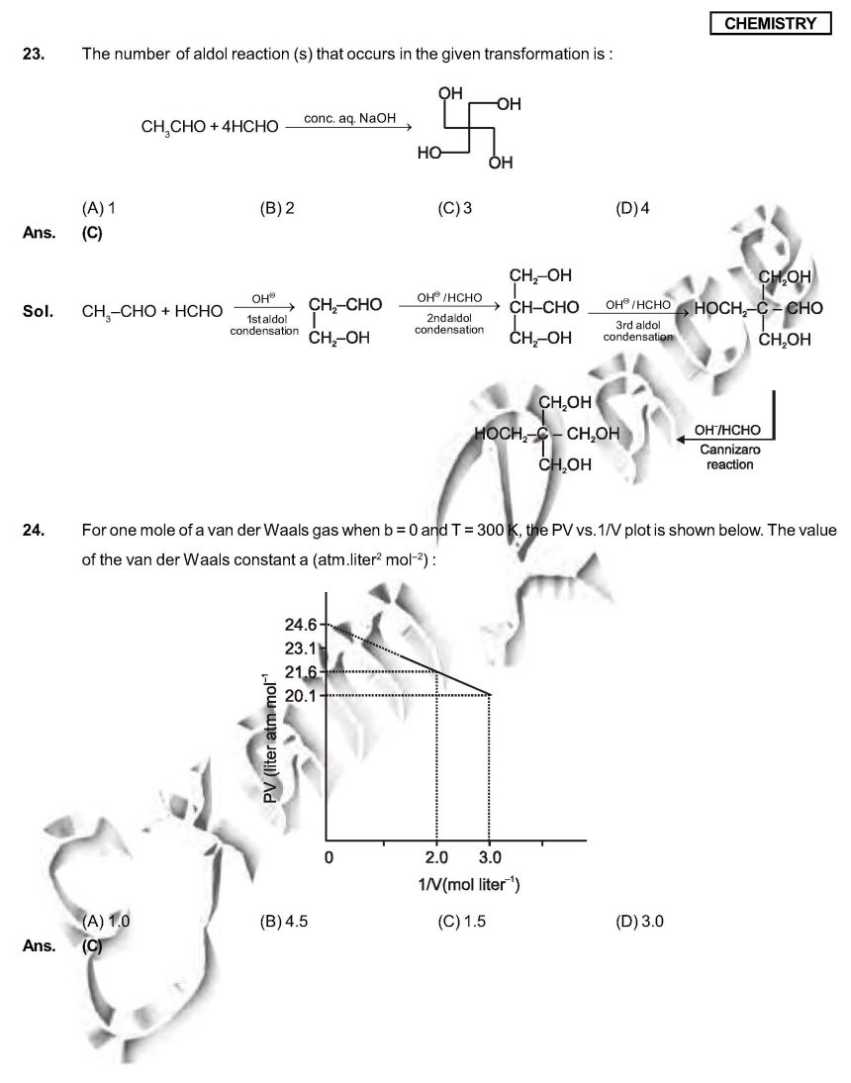 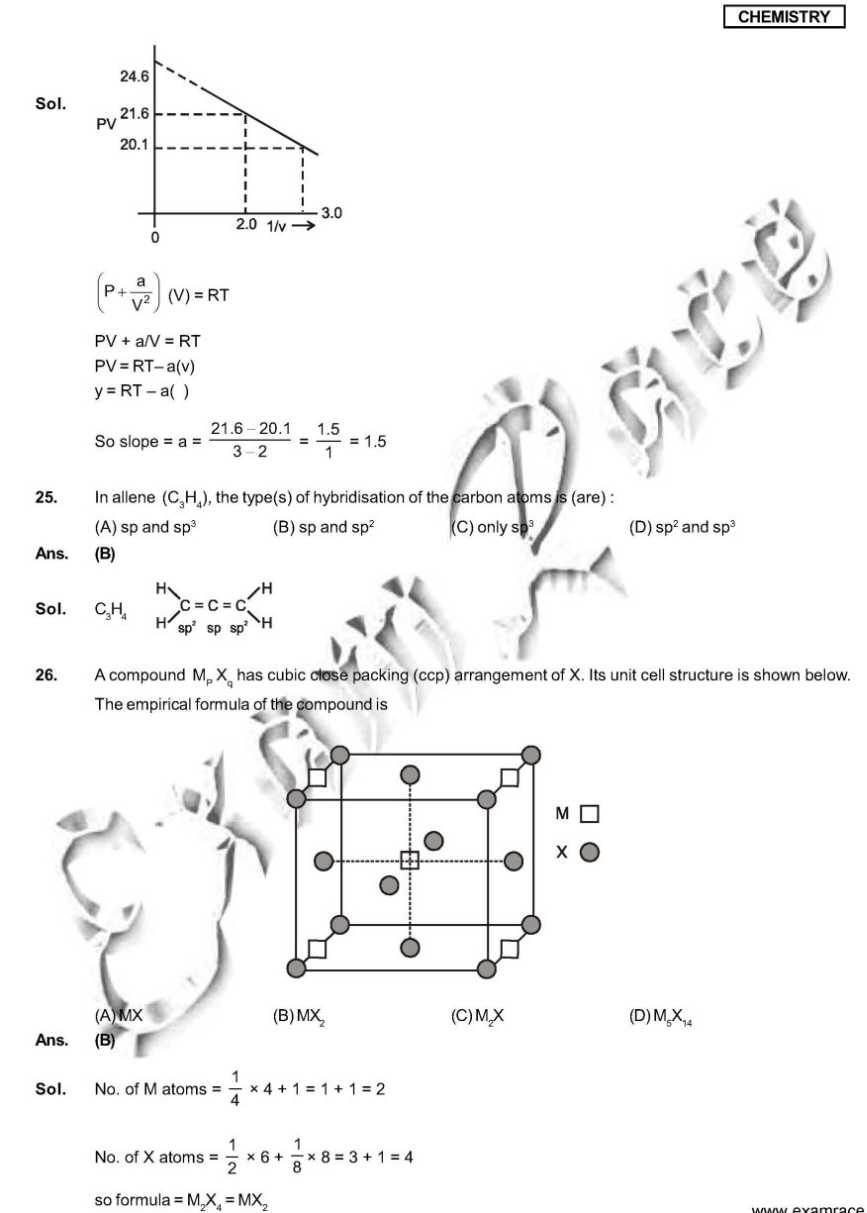 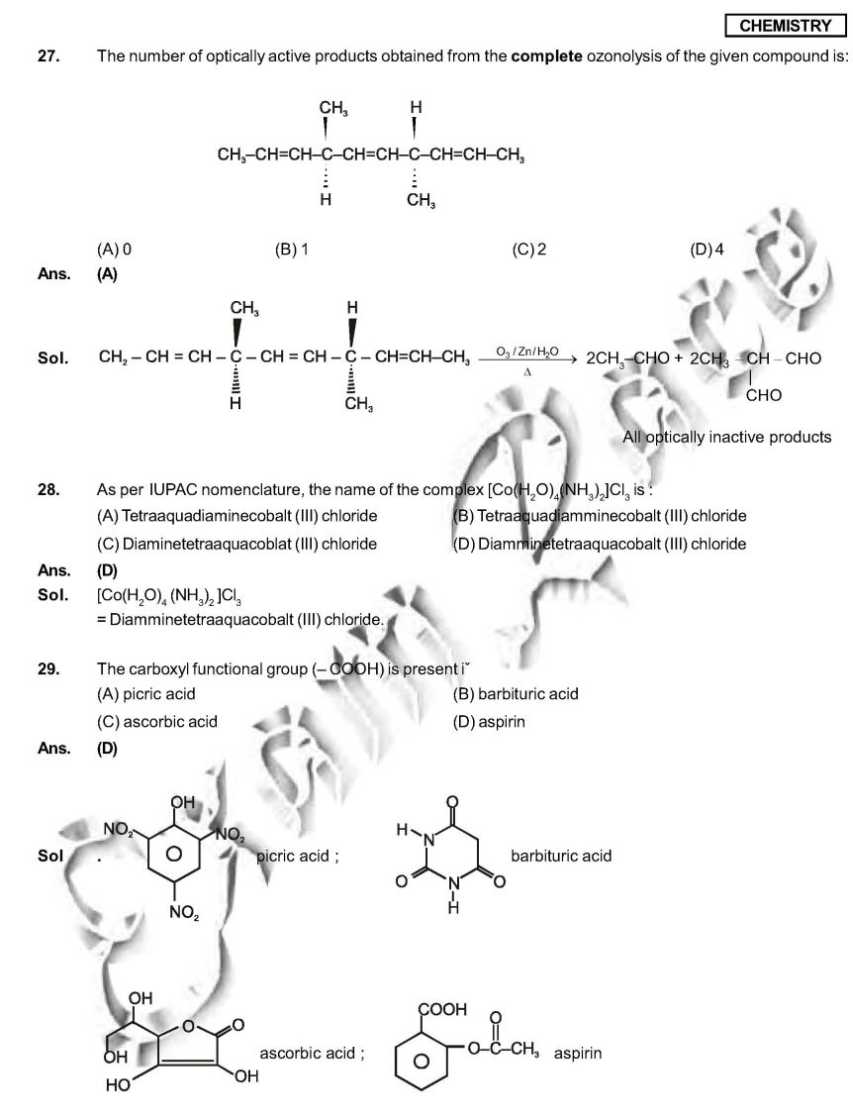 Here is the attachment of the paper..............................
__________________ Answered By StudyChaCha Member |