|
#1
| |||
| |||
|
WILL you please give me question paper for the IIT JAM Chemistry examination ?? As you want to get IIT JAM Chemistry Question Paper so here I am giving you some questions of that paper: If the values of Madelung constants of the following compounds are equal, then their lattice energy values decrease in the order (A) KCl > NaF > CaO > Al2O3 (B) Al2O3 > CaO > NaF > KCl (C) NaF > KCl > CaO > Al2O3 (D) Al2O3 > CaO > KCl > NaF The fluoride, whose value of dipole moment is NOT equal to zero, is (A) XeF4 (B) CF4 (C) SF4 (D) PF5 The decreasing order of the ionic nature of the following compounds is (A) LiI > NaBr > KCl > CsF (B) LiI > KCl > NaBr > CsF (C) CsF > NaBr > KCl > LiI (D) CsF > KCl > NaBr > LiI The atomicity and the total number of bonds in the elemental white phosphorus molecule are, respectively, (A) 4 and 6 (B) 6 and 4 (C) 4 and 4 (D) 6 and 6 The octahedral crystal field splitting (Δo) of d orbital energies of the following metal ions decreases in the order (A) Co2+ > Co3+ > Rh3+ (B) Rh3+ > Co3+ > Co2+ (C) Rh3+ > Co2+ > Co3+ (D) Co3+ > Co2+ > Rh3+ The half-life of a radioactive nuclide is 20 years. If a sample of this nuclide has an activity of 6400 disintegrations per minute (dis/min) today, its activity (dis/min) after 100 years would be (A) 850 (B) 1600 (C) 200 (D) 400 The average value of C-C bond order in graphite is (A) 1 (B) 3/2 (C) 3/4 (D) 4/3 The optical absorption spectrum of [Ti(H2O)6]3+ has its absorption maximum at 20300 cm-1. The magnitude of crystal field stabilization energy in cm-1 is (A) 8120 (B) 16240 (C) 24360 (D) 50750 In inorganic qualitative analysis, H2S in acidic medium will NOT precipitate (A) HgS (B) ZnS (C) CuS (D) CdS IIT JAM Chemistry Question Paper  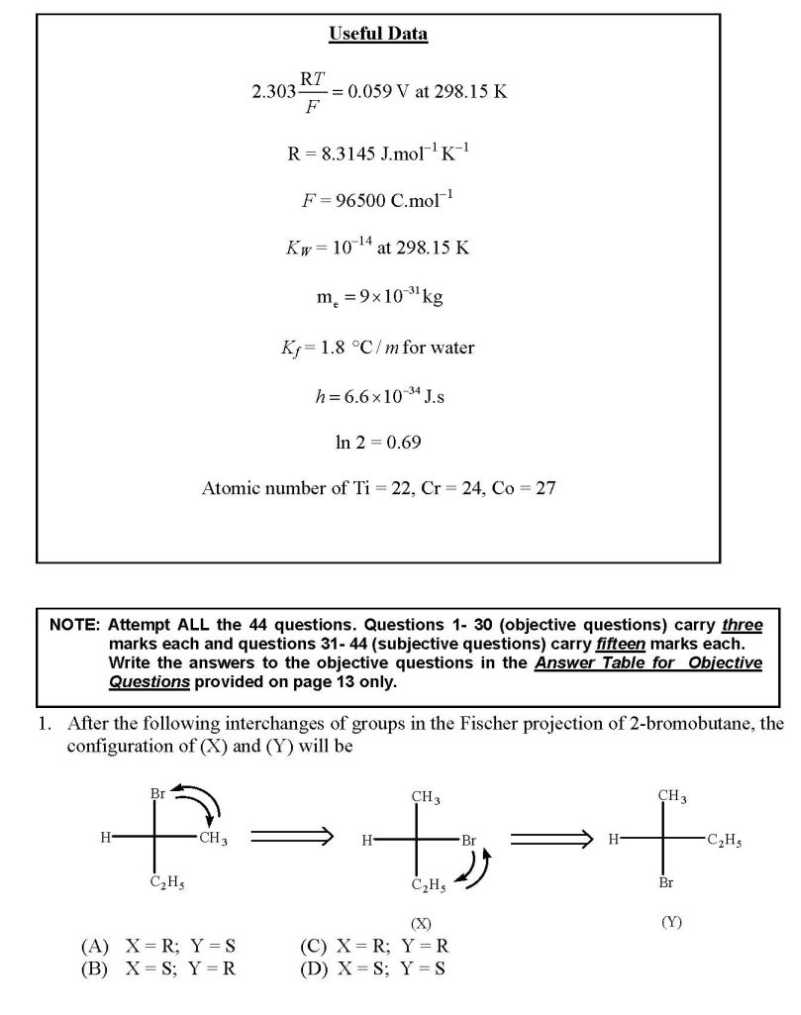 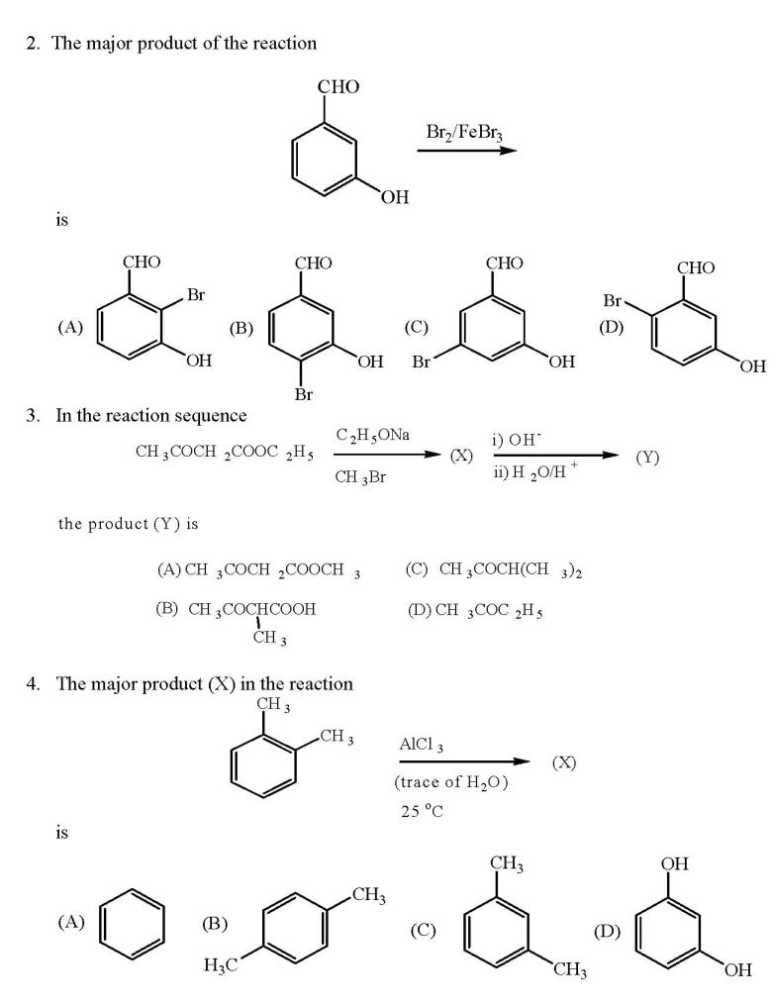 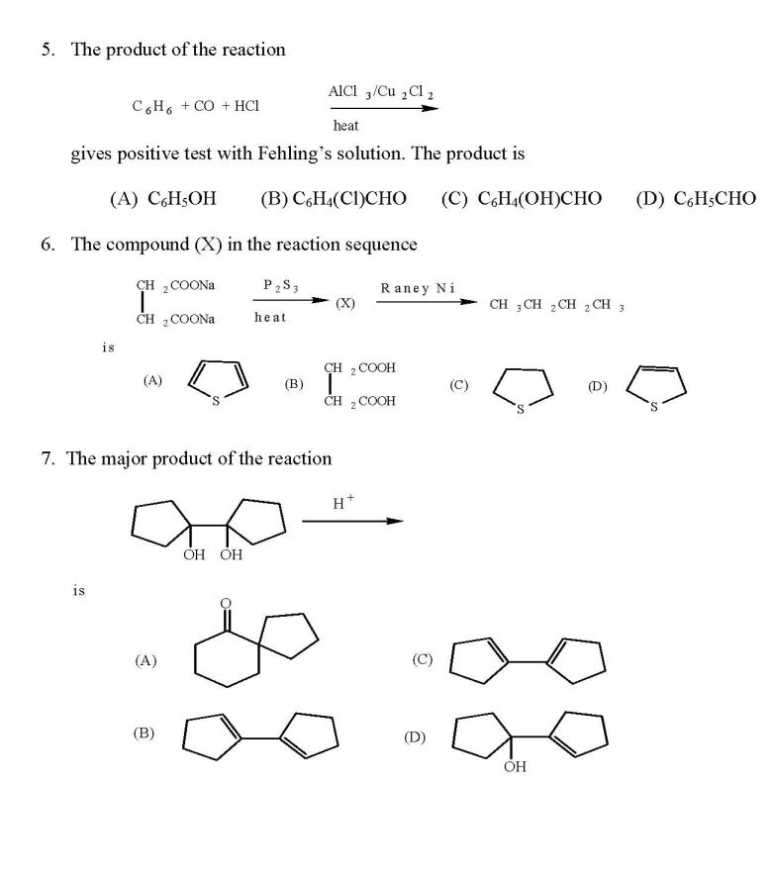 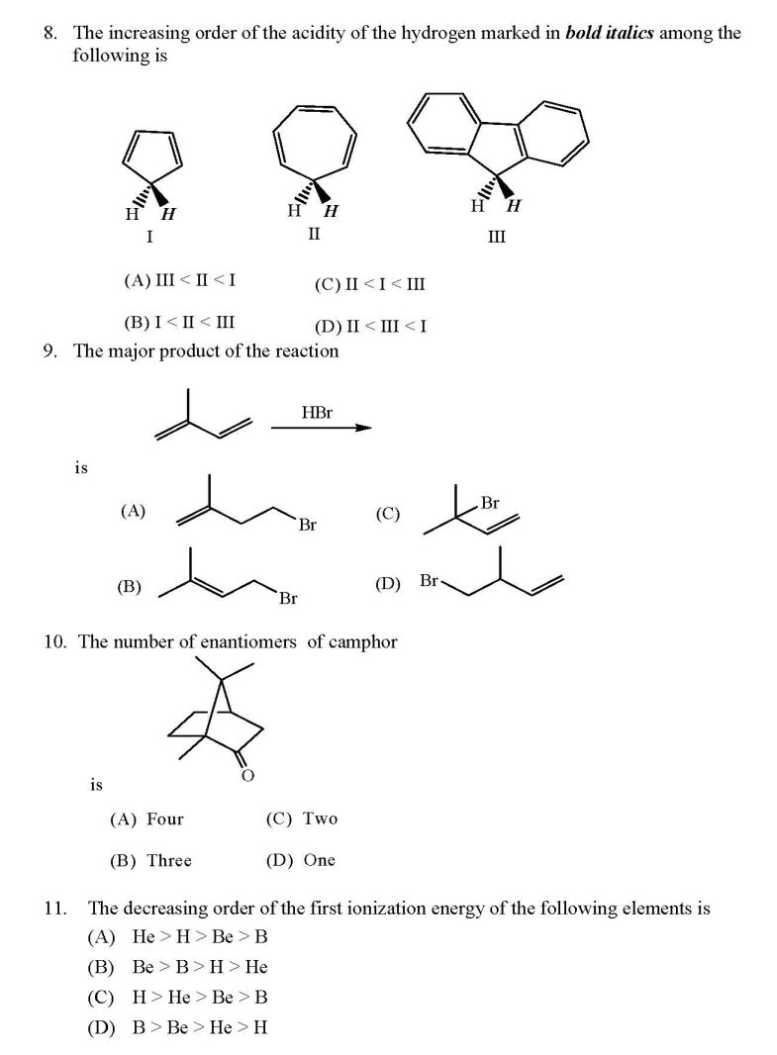 For full question paper here is the attachment.......................... Last edited by Aakashd; October 14th, 2019 at 01:21 PM. |
|
#2
| |||
| |||
|
Here I am giving you question paper for the IIT JAM Chemistry examination in PDF files attached with it so you can get it easily.. Some questions are given below : Q.1 Molecular shape of SOCl2 is (A) square planar (B) trigonal pyramidal (C) triangular planar (D) T-shaped Q.2 Number of three-centre two-electron (3c–2e) bonds present in diborane is (A) 2 (B) 4 (C) 6 (D) 8 Q.5 Number of moles of ions produced by complete dissociation of one mole of Mohr’s salt in water is (A) 3 (B) 4 (C) 5 (D) 6 Q.6 The tetrachloro complexes of Ni(II) and Pd(II) respectively, are (atomic numbers of Ni and Pd are 28 and 46 respectively) (A) diamagnetic and diamagnetic (B) paramagnetic and paramagnetic (C) diamagnetic and paramagnetic (D) paramagnetic and diamagnetic The half-life of any zero-order reaction is (A) independent of concentration (B) proportional to inverse of concentration (C) proportional to concentration (D) proportional to square of the concentration (a) Draw the unit cell structure of NaCl. Calculate the limiting radius ratio of any ionic solid having NaCl like structure. (9) (b) Give molecular formula and structure of the compound formed by reaction of Be(OH)2 with acetic acid.
__________________ Answered By StudyChaCha Member |